Product
Safety and effectiveness are the top considerations for medical devices. As such, stringent sterilization standards are in place against bacteria and viruses on the device’s surface and the demand for sterilized single-used devices is relatively high.
In the past, most medical devices were manufactured from metal for easier sterilization. However, they are heavy and expensive to produce, making metals less favorable to use. As a result, they are gradually being replaced by plastic materials.
The suitability of plastic materials for medical applications primarily depends on whether or not the materials can withstand the sterilization process. The most common types of sterilization are heat, ethylene oxide (EtO), and gamma radiation. Different material grades are designed to meet various sterilization processes to ensure product safety and effectiveness.
TPEs Material Demonstrate Temperature Tolerance, High Fatigue Resistance, and Good Compatibility with Polypropylene (PP)
Medical devices need to undergo heat, gamma rays, or chemical sterilization processes; TPEs do not easily oxidize and degrade, and do not become brittle in low temperatures, making them suitable materials for medical devices or products that need to be in cryogenic storage.
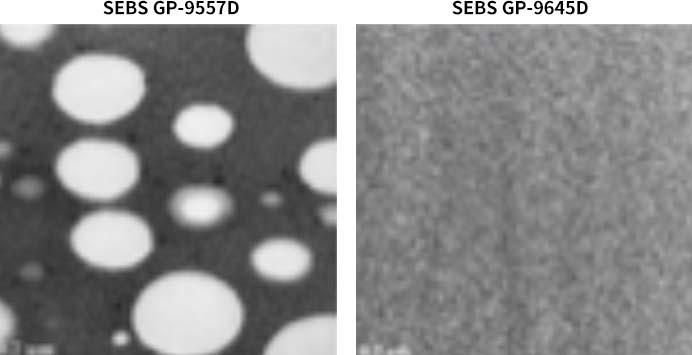
Due to TPEs’ good compatibility with polypropylene (PP), a lesser amount of TPEs is to be added during the material modification process to achieve the desired result, which enables the optimization of the manufacturing process and improved manufacturing efficiency. Among them, SEBS grade GP-9645D with high content of vinyl structure has the best dispersion and compatibility performance:
TPE grades such as SBS (dry) and SEBS have high flow and low hardness properties and can be used in non-oil-extended manufacturing processes. The use of the aforementioned TPE grades can ensure that the products are not at risk of oil precipitation and can enhance overall product safety.
TPEs’ high transparency, softness, and excellent processability contribute to TPEs application in medical tubings, such as catheters and tubing for fluid or gas flow. Currently, TPEs are most commonly used in infusion tubes, infusion bags, urinary catheters, urinary bags, medical mattresses, wheelchair grips, syringe plugs, and face masks.
Select Regulatory Compliant TPE Materials According to the Application Range
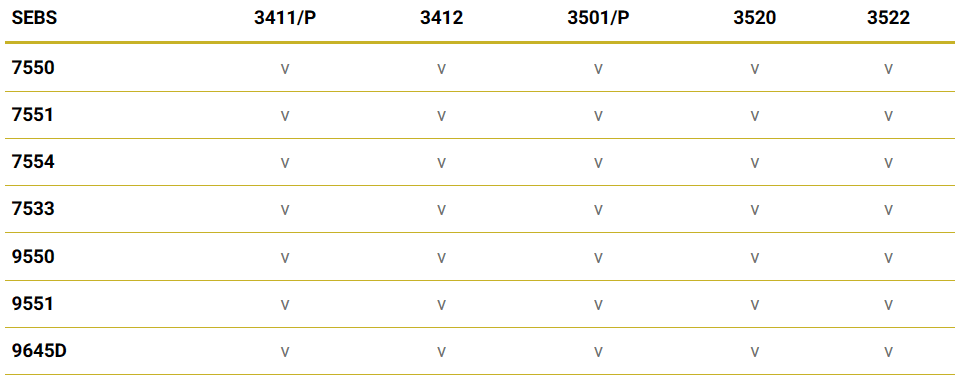
LCY’s TPEs have passed international standard requirements such as FDA and USP 88 Class VI tests, ensuring that materials used in medical application processing are stable and safe. The following table offers an overview of our product grade inspections: